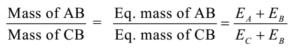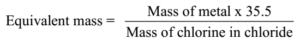Science > Chemistry > Concept of Atomic Mass and Equivalent Mass > Double Displacement Method In the last two articles, we have studied the hydrogen displacement method, oxide formation method, reduction method, and chloride formation method to determine the equivalent mass of metal. In this article, we shall study double displacement method and metal displacement […]