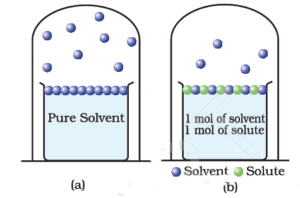
Science > Chemistry > Solutions and Their Colligative Properties > Numerical Problems on Lowering of Vapour Pressure In this article, we shall study to solve problems based on relative lowering of vapour pressure and to calculate the molecular mass of a solute. Example – 01: The vapour pressure of a pure liquid at 298K is […]