Science > Chemistry > Solutions and Their Colligative Properties > Numerical Problems on Molality
In this article, we shall study numerical problems to calculate molality of a solution.
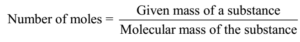
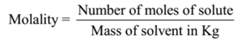

Example – 01:
7.45 g of potassium chloride (KCl) was dissolved in 100 g of water. Calculate the molality of the solution.
Given: mass of solute (KCl) = 7.45 g, mass of solvent (water) = 100 g = 0.1 kg
To Find: Molarity of solution =?
Solution:
Molecular mass of KCl = 39 g x 1 + 35.5 g x 1 = 74.5 g mol-1
Number of moles of solute (KCl) = given mass/ molecular mass
Number of moles of solute (KCl) = 7.45 g/ 74.5 g mol-1 = 0.1 mol
Molality = Number of moles of solute/Mass of solvent in kg
Molality = 0.1 mol /0.1 kg = 1 mol kg-1
Ans: The molality of solution is 1 mol kg-1 or 1 m.
Example – 02:
11.11 g of urea (NH2CONH2) was dissolved in 100 g of water. Calculate the molarity and molality of the solution. Given N = 14, H = 1, C = 12, O = 16.
Given: mass of solute (urea) = 11.11 g, mass of solvent (water) = 100 g = 0.1 kg
To Find: Molarity of solution =?
Solution:
Molecular mass of urea (NH2CONH2) = 14 g x 2 + 1 g x 4 + 12 g x 1 + 16 g x 1
Molecular mass of urea (NH2CONH2) = 60 g mol-1
Number of moles of solute (urea) = given mass/ molecular mass
Number of moles of solute (urea) = 11.11 g/ 60 g mol-1 = 0.1852 mol
Volume of water = mass of water/ density = 100 g/1 g mL-1 = 100 mL = 0.1 L
Molarity = Number of moles of solute/Volume of solution in L
Molarity = 0.1852 mol /0.1 L = 1.852 mol L-1 or 1.852 mol dm-3
Molality = Number of moles of solute/Mass of solvent in kg
Molality = 0.1852 mol /0.1 kg = 1.852 mol kg-1
Ans: The molarity of solution is 1.852 mol L-1 and the molality is 1.852 mol kg-1
Example – 03:
34.2 g of sugar was dissolved in water to produce 214.2 g of sugar syrup. Calculate molality and mole fraction of sugar in the syrup. Given C = 12, H = 1 and O = 16.
Given: Mass of solute (sugar) = 34.2 g, Mass of solution (sugar syrup) = 214.2 g
To Find: Molality and mole fraction =?
Solution:
Mass of Solution = Mass of solute + mass of solvent
Mass of solvent = mass of solution – mass of solute = 214.2 g – 34.2 g = 180 g = 0.180 kg
Molar mass of sugar (C12H22O11) = 12 g x 12 + 1 g x 22 + 16 g x 11 = 342 g mol-1
Number of moles of solute (sugar) = nB = Given mass/ molecular mass = 34.2 g/342 g mol-1 = 0.1 mol
Molality = Number of moles of solute/Mass of solvent in kg
Molality = 0.1 mol /0.180 kg = 0.5556 mol kg-1
Molar mass of water (H2O) = 1 g x 2 + 16 g x 1 = 18 g mol-1
Number of moles of solvent (water) = nA = Given mass/ molecular mass = 180 g/18 g mol-1 = 10 mol
Total number of moles = nA + nB = 0.1 + 10 = 10.1 mol
Mole fraction of solute (sugarl) = xB = nB/(nA + nB) = 0.1/10.1 = 0.0099
Mole fraction of sugar = 0.0099
Ans: Molality of solution = 0.5556 mol kg-1 and mole fraction of sugar = 0.0099
Example – 04:
10.0 g KCl is dissolved in 1000 g of water. If the density of the solution is 0.997 g cm-3, calculate a) molarity and b) molality of the solution. Atomic masses K = 39 g mol-1, Cl = 35.5 g mol-1.
Given: the mass of solute (KCl) = 10 g, the mass of solvent (water) = 1000 g = 1 kg, density of solution = 0.997 g cm-3,
To Find: molarity =? molality = ?
Solution:
Molecular mass of KCl = 39 g x 1 + 35.5 g x 1 = 74.5 g mol-1
Number of moles of solute (KCl) = given mass/ molecular mass
Number of moles of solute (KCl) = 10 g/ 74.5 g mol-1 = 0.1342 mol
Molality = Number of moles of solute/Mass of solvent in kg
Molality = 0.1342 mol /1 kg = 0.1342 mol kg-1
Mass of solution = 10 g + 1000 g = 1010 g
Volume of solution = mass of solution/density = 1010/0.997 g cm-3
Volume of solution = 1013 cm3 = 1013 mL = 1.013 L
Molarity = Number of moles of solute/Volume of solution in L
Molarity = 0.1342 mol /1.013 L = 0.1325 mol L-1
Ans: The molarity of the solution is 0.1325 mol L-1 or 0.1325 M, the molality of the solution is 0.1342 mol kg-1 or 0.1342 m.
Example – 05:
Calculate molarity and molality of the sulphuric acid solution of density 1.198 g cm-3 containing 27 % by mass of sulphuric acid.
Given: density of the solution = 1.198 g cm-3, % mass of sulphuric acid = 27%,
To Find: Molarity =? and molality =?
Solution:
Consider 100 g of solution
Mass of H2SO4 = 27 g and mass of H2O = 100 – 27 g = 73 g = 0.073 kg
Molecular mass H2SO4 = 1 g x 2 + 32 g x 1 + 16g x 4 = 98 g mol-1
Number of moles of H2SO4 = nB = 27 g/ 98 g = 0.2755 mol
Density of solution = 1.198 g cm-3
Volume of solution = Mass of solution / density = 100 g /1.198 g cm-3 = 83.47 cm3 = 83.47 mL = 0.08347 L
Molarity of solution = Number of moles of the solute/volume of solution in L = 0.2755/0.08347 = 3.301 M
Molality = Number of moles of solute/mass of sovent in kg
Molality = 0.2755 mol /0.073 kg = 3.774 mol L-1
Ans: The molarity of solution is 3.374 mol L-1 or 3.374 M, the molality of solution is 3.774 mol L-1 or 3.774 m
Example – 06:
Calculate the mole fraction, molality and molarity of HNO3 in a solution containing 12.2 % HNO3. Given density of HNO3 as 1.038 g cm-3, H = 1, N = 14, O = 16.
Given: density of the solution = 1.038 g cm-3, % mass of HNO3 = 12.2 %,
To Find: mole fraction =? molarity =? and molality =?
Solution:
Consider 100 g of solution
Mass of HNO3 = 12.2 g and mass of H2O = 100 – 12.2 g = 87.8 g = 0.0878 kg
Molecular mass of water (H2O) = 1 g x 2 + 16 g x 1 = 18 g mol-1
Molecular mass HNO3 = 1 g x 1 + 14 g x 1 + 16g x 3 = 63 g mol-1
Number of moles of water = nA = 87.8 g/ 18 g = 4.8778 mol
Number of moles of HNO3 = nB = 12.2 g/ 63 g = 0.1937 mol
Total number of moles = nA + nB + nC = 4.8778 + 0.1937 = 5.0715
Mole fraction of HNO3 = xB = nB/(nA +nB) = 0.1937/5.0715 = 0.0382
Density of solution = 1.038 g cm-3
Volume of solution = Mass of solution / density = 100 g /1.038 g cm-3 = 96.34 cm3 = 96.34 mL = 0.09634 L
Molarity of solution = Number of moles of the solute/volume of solution in L = 0.1937/0.09634 =2.011 M
Molality = Number of moles of solute/mass of sovent in kg
Molality = 0.1937 mol /0.0878 kg = 2.206 mol kg-1
Ans: The mole fraction of HNO3 is 0. 0382, the molarity of solution is 2.011 mol L-1 or 2.011 M, the molality of solution is 2.206 mol kg-1 or 2.206 m
Example – 07:
Calculate molarity and molality of 6.3 % solution of nitric acid having density 1.04 g cm-3. Given atomic masses H = 1, N = 14 and O = 16.
Given: density of the solution = 1.04 g cm-3, % mass of HNO3 = 6.3 %,
To Find: mole fraction =? molarity =? and molality =?
Solution:
Consider 100 g of solution
Mass of HNO3 = 6.3 g and mass of H2O = 100 – 6.3 g = 93.7 g = 0.0937 kg
Molecular mass of water (H2O) = 1 g x 2 + 16 g x 1 = 18 g mol-1
Molecular mass HNO3 = 1 g x 1 + 14 g x 1 + 16g x 3 = 63 g mol-1
Number of moles of water = nA = 93.4 g/ 18 g = 5.189 mol
Number of moles of HNO3 = nB = 6.3 g/ 63 g = 0.1 mol
Density of solution = 1.04 g cm-3
Volume of solution = Mass of solution / density = 100 g /1.04 g cm-3 = 96.15 cm3 = 96.15 mL = 0.09615 L
Molarity of solution = Number of moles of the solute/volume of solution in L = 0.1/0.09615 =1.040 M
Molality = Number of moles of solute/mass of sovent in kg
Molality = 0.1 mol /0.0937 kg = 1.067 mol kg-1
Ans: The molarity of solution is 1.040 mol L-1 or 1.040 M
The molality of solution is 1.067 mol kg-1 or 1.067 m
Example – 08:
An aqueous solution of NaOH is marked 10% (w/w). The density of the solution is 1.070 g cm-3. Calculate molarity, molality and mole fraction of NaOH in water. Given Na = 23, H =1 , O = 16
Given: density of the solution = 1.038 g cm-3, % mass of HNO3 = 12.2 %,
To Find: mole fraction =? molarity =? and molality =?
Solution:
Consider 100 g of solution
Mass of NaOH = 10 g and mass of H2O = 100 – 10 g = 90 g = 0.090 kg
Molecular mass of water (H2O) = 1 g x 2 + 16 g x 1 = 18 g mol-1
Molecular mass NaOH = 23 g x 1 + 16 g x 1 + 1 g x 1 = 40 g mol-1
Number of moles of water = nA = 90 g/ 18 g = 5 mol
Number of moles of NaOH = nB = 10 g/ 40 g = 0.25 mol
Total number of moles = nA + nB = 5 + 0.25 = 5.25 mol
Mole fraction of NaOH = xB = nB/(nA +nB) = 0.25/5.25 = 0.0476
Density of solution = 1.070 g cm-3
Volume of solution = Mass of solution / density = 100 g /1.070 g cm-3 = 93.46 cm3 = 93.46 mL = 0.09346 L
Molarity of solution = Number of moles of the solute/volume of solution in L = 0.25/0.09346 =2.675 M
Molality = Number of moles of solute/mass of sovent in kg
Molality = 0.25 mol /0.090 kg = 2.778 mol kg-1
Ans: The molarity of solution is 2.675mol L-1 or 2.675 M, the molality of solution is 2.778 mol kg-1 or 2.778 m, the mole fraction of NaOH is 0. 0476
Example – 09:
A solution of glucose in water is labelled as 10 % (w/w). Calculate a) molality and b) molarity of the solution. Given the density of the solution is 1.20 g mL-1 and molar mass of glucose is 180 g mol-1.
Given: density of the solution = 1.20 g cm-3, % mass of glucose = 10 %, molar mass of glucose is 180 g mol-1.
To Find: molarity =? and molality =?
Solution:
Consider 100 g of solution
Mass of glucose = 10 g and mass of H2O = 100 – 10 g = 90 g = 0.090 kg
Molecular mass of water (H2O) = 1 g x 2 + 16 g x 1 = 18 g mol-1
Molecular mass glucose = 180 g mol-1
Number of moles of water = nA = 90 g/ 18 g = 5 mol
Number of moles of glucose = nB = 10 g/ 180 g = 0.0556 mol
Density of solution = 1.20 g cm-3
Volume of solution = Mass of solution / density = 100 g /1.20 g cm-3 = 83.33 cm3 = 83.33 mL = 0.08333 L
Molarity of solution = Number of moles of the solute/volume of solution in L = 0.0556/0.08333 =0.6672 M
Molality = Number of moles of solute/mass of sovent in kg
Molality = 0.0556 mol /0.090 kg = 0.6178 mol kg-1
Ans: The molarity of solution is 0.6672 mol L-1 or 0.6672 M, the molality of solution is 0.6178 mol kg-1 or 0.6178 m,
Example – 10:
Battery acid 4.22 M aqueous H2SO4 solution, and has density 1.21 g cm-3. What is the molality of H2SO4. Given H = 1, S = 32, O = 16
Given: density of the solution = 1.21 g cm-3, Molarity of solution = 4.22 M.
To Find: molality =?
Solution:
Let us consider 1 L of solution
Molecular mass H2SO4 = 1 g x 2 + 32 g x 1 + 16g x 4 = 98 g mol-1
Molarity of solution = Number of moles of the solute/volume of solution in L
Number of moles of solute = Molarity of solution x volume of solution in L = 4.22 x 1 = 4.22
Density of solution = 1.21 g cm-3 = 1.21 g/mL = 1.21 x 103 g/L = 1.21 kg/L
Mass of solution = Volume of solution x density = 1 L x 1.21 kg/L = 1.21 kg
Mass of solute (H2SO4) = Number of moles x molecular mass = 4.22 x 98
Mass of solute (H2SO4) = 413.56 g = 0.41356 kg
Mass of solvent = mass of solution – mass of solute = 1.21 – 0.41356 = 0.79644 kg
Molality = Number of moles of solute/mass of sovent in kg
Molality = 4.22 mol /0.79644 kg = 5.298 mol kg-1
Ans: Molality of solution is 5.298 mol kg-1 or 5.298 m
Example – 11:
The density of 5.35 M H2SO4 solution is 1.22 g cm-3. What is molality of a solution?
Given: density of the solution = 1.22 g cm-3, Molarity of solution = 5.35 M.
To Find: molality =?
Solution:
Let us consider 1 L of solution
Molecular mass H2SO4 = 1 g x 2 + 32 g x 1 + 16g x 4 = 98 g mol-1
Molarity of solution = Number of moles of the solute/volume of solution in L
Number of moles of solute = Molarity of solution x volume of solution in L = 5.35 x 1 = 5.35
Density of solution = 1.22 g cm-3 = 1.22 g/mL = 1.22 x 103 g/L = 1.22 kg/L
Mass of solution = Volume of solution x density = 1 L x 1.22 kg/L = 1.22 kg
Mass of solute (H2SO4) = Number of moles x molecular mass = 5.35 x 98
Mass of solute (H2SO4) = 524.3 g = 0.5243 kg
Mass of solvent = mass of solution – mass of solute = 1.22 – 0.5243 = 0.6957 kg
Molality = Number of moles of solute/mass of sovent in kg
Molality = 5.35 mol /0.6957 kg = 7.690 mol kg-1
Ans: Molality of solution is 7.690 mol kg-1 or 7.690 m
Example – 12:
Calculate the mole fraction of solute in its 2 molal aqueous solution.
Given: molality = 2 molal
To Find: Mole fraction =?
Solution:
Molecular mass of water (H2O) = 1 g x 2 + 16 g x 1 = 18 g mol-1
Molality of solution = 2 molal = 2 mol mol kg-1
The number of moles of solute = 2
The mass of solvent (water) = 1 kg = 1000 g
Number of moles of solvent (water) = 1000/16 = 55.55
Mole fraction of solute = 2/(2 + 55.55) = 2/57.55 = 0.03475
Ans: Mole fraction of solute is 0.0345
Previous Topic: Numerical Problems on Molarity
Next Topic: Short Cut Methods to Calculate Concentration of Solution

23 replies on “Numerical Problems on Molality”
Where you found this type of questions ? Pls reply me
Past State Boards, CBSE, JEE and NEET Papers
thanks this is very helpful
Wooww, I love these questions
They upgrade your thinking ability
Nice question
wow
Really its very helpful for us thank you so much
These questions help me but I need questions of mole fraction
Problems on Mole Fraction
https://thefactfactor.com/facts/pure_science/chemistry/physical-chemistry/mole-fraction/7855/
More Topics in Colligative Properties:
https://thefactfactor.com/chemistry/solutions-and-their-colligative-properties/
More Topics in Chemistry:
https://thefactfactor.com/chemistry/
Love this question
Best thinking upgrade questions
wow
Nice questions really help me alot 👍👍👍👌👌
Its a nice questions and not to hard
Thanx for questions……😊👍
These questions help to improve myself 😇😇 so thnx for this question 🤗🤗🤗🤗🤗
Thank you for these questions.
This was very helpful ☺️
Thanks a lot.This help me so much
Thank u so much
Thanks a lot, the content is so helpful
Very bralint questions
I love you 😘😘😘
Thankyou so much
Thank you so much.
Very helpful.