Science > Chemistry > Solutions and Their Colligative Properties > Numerical Problems on Molarity
In this article, we shall study numerical problems to calculate the molarity of a given solution.
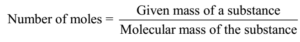

Example – 01:
A solution of NaOH (molar mass 40 g mol-1) was prepared by dissolving 1.6 g of NaOH in 500 cm3 of water. Calculate molarity of the NaOH solution.
Given: Mass of NaOH = 1.6 g, molar mass of NaOH = 40 g mol-1, volume of water = 500 cm3 = 500 mL = 0.5 L
To Find: Molarity =?
Solution:
Number of moles = Given mass/ Molecular mass = 1.6 g/40 g mol-1 = 0.04 mol
Molarity = Number of moles of solute/Volume of solution in L
Molarity = 0.04 mol /0.5 L = 0.08 mol L-1
Ans: The molarity of NaOH solution is 0.08 mol L-1 or 0.08 M
Example – 02:
Calculate molarity of 4 g caustic soda dissolved in 200 mL of solution.
Given: Mass of solute (caustic soda) = 4 g, volume of solution = 200 mL = 0.2 L
To Find: Molarity of the solution =?
Solution:
Molar mass of caustic solute (caustic soda NaOH) = 23 g x1 + 16 g x 1 + 1 g x 1 = (23 + 16 + 1) g = 40 g
Number of moles of caustic solute (caustic soda) = given mass/molecular mass = 4 g/ 40 g = 0.1
Molarity of solution = Number of moles of the solute/volume of solution in L = 0.1/0.2 = 0.5 M
Ans: The molarity of caustic soa solution is 0.5 mol L-1 or 0.5 M
Example – 03:
Calculate molarity of 5.3 g anhydrous sodium carbonate dissolved in 100 mL of solution.
Given: Mass of solute (sodium carbonate) = 5.3 g, volume of solution = 100 mL = 0.1 L
To Find: Molarity of the solution =?
Solution:
Molar mass of (Na2CO3) = 23 g x 2 + 12 g x 1 + 16 g x 3 = (46 + 12 + 48) g = 106 g
Number of moles of (Na2CO3) = given mass/molecular mass = 5.3 g/ 106 g = 0.05
Molarity of solution = Number of moles of the solute/volume of solution in L = 0.05/0.1 = 0.5 M
Ans: The molarity of sodium carbonate solution is 0.5 mol L-1 or 0.5 M
Example – 04:
Calculate molarity of 0.365 g pure HCl gas dissolved in 50 mL of solution.
Given: Mass of solute (HCl) = 0.365 g, volume of solution = 50 mL = 0.05 L
To Find: Molarity of the solution =?
Solution:
Molar mass of HCl = 1 g x 1 + 35.5 g x 1 = (1 + 35.5) g = 36.5 g
Number of moles of HCl = given mass/molecular mass = 0.365 g/ 36.5 g = 0.01
Molarity of solution = Number of moles of the solute/volume of solution in L = 0.01/0.05 = 0.2 M
Ans: The molarity of HCl solution is 0.2 mol L-1 or 0.2 M
Example – 05:
Calculate molarity of 5.85 g NaCl dissolved in 200 mL of solution.
Given: Mass of solute (NaCl) = 5.85 g, volume of solution = 200 mL = 0.2 L
To Find: Molarity of the solution =?
Solution:
Molar mass of NaCl = 23 g x 1 + 35.5 g x 1 = (23 + 35.5) g = 58.5 g
Number of moles of NaCl = given mass/molecular mass = 5.85 g/ 58.5 g = 0.1
Molarity of solution = Number of moles of the solute/volume of solution in L = 0.1/0.2 = 0.5 M
Ans: The molarity of NaCl solution is 0.5 mol L-1 or 0.5 M
Example – 06:
Calculate molarity of 20.6 g NaBr dissolved in 500 mL of solution.
Given: Mass of solute (NaCl) = 20.6 g, volume of solution = 500 mL = 0.5 L
To Find: Molarity of the solution =?
Solution:
Molar mass of NaBr = 23 g x 1 + 80 g x 1 = (23 + 80) g = 103 g
Number of moles of NaBr = given mass/molecular mass = 20.6 g/ 103 g = 0.2
Molarity of solution = Number of moles of the solute/volume of solution in L = 0.2/0.5 = 0.4 M
Ans: The molarity of NaBr solution is 0.4 mol L-1 or 0.4 M
Example – 07:
Calculate molarity of pure water if its density is 1 g/mL.
Given: Density of water = 1 g/mL
To Find: Molarity of pure water =?
Solution:
Let us consider 1000 mL of water
Mass of water = volume x density = 1000 mL x 1 g/mL = 1000 g
Molar mass of water = 1 g x 2 + 16 g x 1 = (2 + 16) g = 18 g
Number of moles of water = given mass/molecular mass = 1000/ 18 g = 55.5
Molarity of pure water = Number of moles of the solute/volume of solution in L = 55.55/1 = 55.55 M
Ans: The molarity of pure water is 55.55 mol L-1 or 55.5 M
Example – 08:
Calculate the quantity of anhydrous sodium carbonate required to produce 250 mL decimolar solution.
Given: volume of solution = 250 mL = 0.25 L, molarity = decimolar = M/10 = 0.1 M
To Find: Mass of anhydrous sodium carbonate =?
Solution:
Molarity = Number of moles of the solute/volume of solution in L
0.1 = Number of moles of the solute/0.25
Number of moles of the solute = 0.1 x 0.25 = 0.025 mol
Molar mass of (Na2CO3) = 23 g x 2 + 12 g x 1 + 16 g x 3 = (46 + 12 + 48) g = 106 g
Number of moles of = given mass/molecular mass
Mass of Na2CO3 = Number of moles x molecular mass
Mass of Na2CO3 = 106 x 0.025 = 2.65 g
Ans: The quantity of sodium carbonate required is 2.65 g
Example – 09:
Sulphuric acid is 95.8 % by mass. Calculate molarity and mole fraction of H2SO4 of density 1.91 g cm-3. Given H = 1, S = 32, O = 16.
Given: % by mass = 95.8 %, Density of solution = 1.91 g cm-3
To Find: Mole fraction =? Molarity =?
Solution:
Consider 100 g of solution
Mass of H2SO4 = 95.8 g and mass of H2O = 100 – 95.8 g = 4.2 g
Molecular mass of water (H2O) = 1 g x 2 + 16 g x 1 = 18 g mol-1
Molecular mass H2SO4 = 1 g x 2 + 32 g x 1 + 16g x 4 = 98 g mol-1
Number of moles of water = nA = 4.2 g/ 18 g = 0.2333 mol
Number of moles of H2SO4 = nB = 95.8 g/ 98 g = 0.9776 mol
Total number of moles = nA + nB + nC = 0.2333 + 0.9776 = 1.2109
Mole fraction of H2SO4 = xB = nB/(nA +nB) = 0.9776/1.2109 = 0.8073
Density of solution = 1.91 g cm-3
Volume of solution = Mass of solution / density = 100 g /1.91 g cm-3 = 52.36 cm3 = 52.36 mL = 0.05236 L
Molarity of solution = Number of moles of the solute/volume of solution in L = 0.9776/0.05236 = 18.67 M
Ans: The mole fraction of H2SO4 is 0.8073 and molarity of solution is 18.67 mol L-1 or 18.67 M
Example – 10:
Commercially available concentrated hydrochloric acid is an aqueous solution containing 38% HCl gas by mass. If its density is 1.1 g cm-3, calculate molarity of HCl solution and also calculate the mole fraction of HCl and H2O.
Given: % by mass = 38 %, Density of solution = 1.1 g cm-3
To Find: Mole fraction =? Molarity =?
Solution:
Consider 100 g of solution
Mass of HCl = 38 g and mass of H2O = 100 – 38 g = 62 g
Molecular mass of water (H2O) = 1 g x 2 + 16 g x 1 = 18 g mol-1
Molecular mass HCl = 1 g x 1 + 35.5 g x 1 = 36.5 g mol-1
Number of moles of water = nA = 62 g/ 18 g = 3.444 mol
Number of moles of HCl = nB = 38 g/ 36.5 g = 1.041 mol
Total number of moles = nA + nB + nC = 3.444 + 1.041 = 4.485
Mole fraction of HCl = xB = nB/(nA +nB) = 1.041/4.485 = 0.2321
Mole fraction of H2O = xA = nA/(nA +nB) = 3.444/4.485 = 0.7679
Density of solution = 1.1 g cm-3
Volume of solution = Mass of solution / density = 100 g /1.1 g cm-3 = 90.91 cm3 = 90.91 mL = 0.09091 L
Molarity of solution = Number of moles of the solute/volume of solution in L = 1.041/0.09091 = 11.45 M
Ans: Molarity of solution is 11.45 mol L-1 or 11.45 M, the molefraction of HCl is 0.2321 and that of H2O is 0.7679
Example – 11:
Commercially available concentrated hydrochloric acid is an aqueous solution containing 40% HCl gas by mass. If its density is 1.2 g cm-3, calculate molarity of HCl solution.
Given: % by mass = 40 %, Density of solution = 1.2 g cm-3
To Find: Molarity =?
Solution:
Consider 100 g of solution
Mass of HCl = 40 g and mass of H2O = 100 – 40 g = 60 g
Molecular mass HCl = 1 g x 1 + 35.5 g x 1 = 36.5 g mol-1
Number of moles of HCl = nB = 40 g/ 36.5 g = 1.096 mol
Density of solution = 1.2 g cm-3
Volume of solution = Mass of solution / density = 100 g /1.2 g cm-3 = 83.33 cm3 = 83.33 mL = 0.08333 L
Molarity of solution = Number of moles of the solute/volume of solution in L = 1.096/0.08333 = 13.15 M
Ans: Molarity of solution is 13.15 mol L-1 or 13.15 M
Example – 12:
Calculate molarity of a solution containing 50 g of NaCl in 500 g of solution and having density 0.936 g/cm3.
Given: Mass of solute (NaCl) = 50 g, mass of solution = 500 g, density of solution = d = 0.936 g/cm3.
To Find: Molarity of solution = M =?
Molar mass of NaCl = 23 g x 1 + 35.5 g x 1 = (23 + 35.5) g = 58.5 g
Number of moles of NaCl = given mass/molecular mass = 50 g/ 58.5 g = 0.8547
Density of solution = 0.936 g cm-3
Volume of solution = Mass of solution / density = 500 g /0.936 g cm-3 = 534.2 cm3 = 534.2 mL = 0.5342 L
Molarity of solution = Number of moles of the solute/volume of solution in L = 0.8547/0.5342 = 1.6 M
Ans: The molarity of NaCl solution is 1.6 mol L-1 or 1.6 M
Previous Topic: Numerical Problems on Mole Fraction
Next Topic: Numerical Problems on Molality

6 replies on “Numerical Problems on Molarity”
This is best website for practice sir please send me more questions for solution chapter
Its so much informative …
Thanks 😊
Thanks alot it’s much much informative
Questions were helpful
thank you very much I like this module question
Thank you