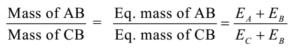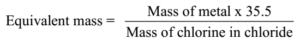Science > Chemistry > Third Row Elements > Introduction The elements are arranged in the order of increasing atomic numbers, in such a way that elements with similar properties fall in the same vertical column of the periodic table. There are eighteen vertical columns known as groups and seven horizontal rows are known as periods. A […]
Categories
Introduction to the Third Row Elements
- Post author By Hemant More
- Post date June 1, 2020
- 1 Comment on Introduction to the Third Row Elements
- Tags Acidic oxides, Amphoteric oxides, Bad conductors, Basic oxides, Boiling point, Chemistry, Covalent solid, Crystal structure, Ductility, Electrical conductivity, Electronic configuration, Good conductors, Heat conductivity, Hydroxy compounds, Inorganic chemistry, Insulators, Ionization enthalpy, Ionization potential, Malleability, Melting point, Metallic character, Metallic luster, Metallic solids, Molecular solid, Non-metallic character, Oxidants, Oxidation, Oxidizing agents, Oxidizing property, Reducing agents, Reducing property, Reductants, Reduction, Semiconductors, Third-row elements