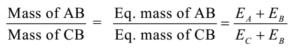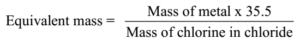Science > Chemistry > Third Row Elements > Metallic Character of Third Row Elements In this article, we shall study the trend im metallic character of third row elements. Metallic Character: The tendency of an atom to lose electrons to form positively charged ion is called its metallic character or electropositive character. Typically metals show […]
Categories
Metallic Character of Third Row Elements
- Post author By Hemant More
- Post date June 1, 2020
- No Comments on Metallic Character of Third Row Elements
- Tags Acidic oxides, Amphoteric oxides, Bad conductors, Basic oxides, Boiling point, Chemistry, Covalent solid, Crystal structure, Ductility, Electrical conductivity, Electronic configuration, Good conductors, Heat conductivity, Hydroxy compounds, Inorganic chemistry, Insulators, Ionization enthalpy, Ionization potential, Malleability, Melting point, Metallic character, Metallic luster, Metallic solids, Molecular solid, Non-metallic character, Oxidants, Oxidation, Oxidizing agents, Oxidizing property, Reducing agents, Reducing property, Reductants, Reduction, Semiconductors, Third-row elements