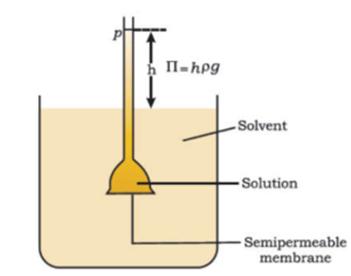
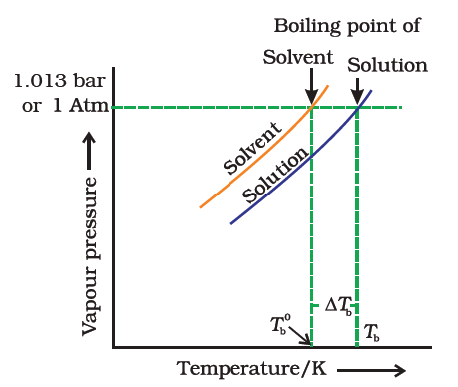
Science > Chemistry > Solid State > Packing in solids In this article, we shall study the different types of close packing of the constituent particles in solids. The Principle of Packing in Solids: In a crystal particle (atoms, molecules or ions) occupy the lattice points in the crystal lattice. These particles may be of […]
Categories
Packing in solids
- Post author By Hemant More
- Post date February 1, 2020
- 9 Comments on Packing in solids
- Tags Atomic mass, Body-centered, Bravais Lattices, Chemistry, Close packing in one dimension, Close packing in three dimensions, Close packing in two dimensions, Coordination number, Crystal lattice, Cubic, Cubic close packing, Cubic structures, Density of solid, Edge length of unit cell, End centered, face-centered, Hexagonal, Hexagonal close packing, Mono Clinic, Neighbouring atom, Octahedral voids, Orthorhombic, Packing efficiency, Packing factor, Simple primitive, Solid-state, Square close packing, Tetragonal, Tetrahedral voids, Triclinic, Type of crystal lattice, Unit cell, Void, Volume of unit cell