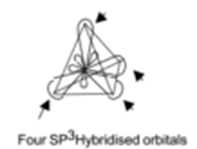
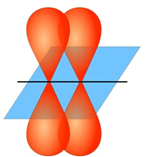
Science > Chemistry > Physical Chemistry > Nature of Chemical Bond > sp Hybridization The mixing of one s – orbital and one p – orbital of the same atom of nearly same energy to form a set of diagonally arranged two identical hybrid orbitals of equivalent energy is called sp hybridization. These hybrid orbitals […]
Categories
SP Hybridization
- Post author By Hemant More
- Post date June 8, 2022
- No Comments on SP Hybridization
- Tags Axial overlap, Chemistry, Covalent bond, Excited state, Formation of Ammonia molecule, Formation of Methane molecule, Formation of water molecule, Geometry of molecule, Ground state, Hybridisation, Hybridization, Hybridized orbitals, Hybridized state, Lateral overlap, Nature of chemical bond, Overlapping of orbitals, P-P overlap, pi bond, S-P overlap, S-S overlap, sigma bond, SP hybridization, SP2 hybridization, SP3 hybridization, Tetrahedral geometry, Valence bond theory