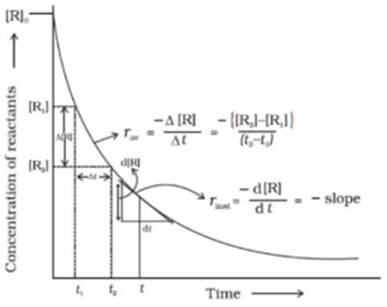
Science > Chemistry > Chemical Kinetics > Arrhenius Equation In this article, we shall study the factors affecting the rate of a chemical reaction and the Arrhenius equation. Factors Affecting the Rate Of Reaction: The Concentration of Reactants: The number of collisions and hence the activated collisions between the reactant molecules increase with the increase […]
Categories
Arrhenius Equation
- Post author By Hemant More
- Post date November 27, 2020
- No Comments on Arrhenius Equation
- Tags Activation energy, Arrhenius Equation, Catalysis, Catalyst, Change of concentration, Change of pressure, Change of temperature, Chemical kinetics, Chemistry, Collision, Collision theory, Concentration, Elementary reactions, First order reaction, Half-Life of reaction, Integrated law, Integrated rate constant, Intensity of light, Molecularity, Multistep reactions, Nature of solvent, No order reaction, Order of reaction, Orientation of Reacting Species, Physical chemistry, Potential energy barrier, Pressure, Rate of reaction, rate-determining step, Reaction intermediates, Surface Area of Reactant, Temperature, Zero order reaction