Science > Chemistry > Atomic Structure > Problems on Calculation of Number of Electrons, Protons, and Neutrons In this article, we shall study to solve problems on the calculations of the number of electrons, protons, and neutrons in atoms, molecules, and species. Example 01: Calculate the charge and mass of 1 mole of electrons. Solution: […]
Category: Pure Sciences
Science > Chemistry > Atomic Structure > Problems Based on Atomic Number, Mass Number, and Neutron Number In this article, we shall study to solve problems based on the calculation of atomic number, atomic mass number, and neutron number Atomic number (Z) : The number of protons (positive charge) present in the nucleus of an […]
Science > Chemistry > Introduction to Organic Chemistry > Empirical and Molecular Formulae of Organic Compounds In this article, we shall study the concept of empirical formula and a molecular formula of a compound. Empirical Formula: The empirical formula of a substance represents the simplest relative whole number ratio of the atoms of each element […]
Science > Chemistry > Introduction to Organic Chemistry > Classification of Organic Compounds In the last artivle we have studied what is organic chemistry? Why it is termed as chemistry of carbon compounds nowaday? In this article we shall study classification of organic compounds. Depending upon the structure organic compounds are classified into two types. […]
A set is a collection of well-defined objects. These objects may be actually listed or may be specified by a rule. In this article, we shall study the application of the definition of a set. Similarly, we shall study to write sets by roster method and set-builder method. Problems on Definition of a Set: Which […]
Science > Chemistry > States of Matter > Charle’s Law Mathematical relationships between volume, pressure, and temperature of a given mass of gas are referred to as Gas laws. In this article. we shall study Pressure-Temperature relation or Gay-Lussac’s law. Gay-Lussac’s Law: Statement: At constant volume the pressure of a given mass of a gas […]
Particle Model of Matter
Science > Chemistry > States of Matter > Introducion A matter is defined as anything that has mass, which occupies space and may be perceived by senses. There are three states of matter, viz. (a) solid, (b) liquid, and (c) gaseous states. Whatever may be the state of matter, it is composed of particles. In […]
Operations on Sets
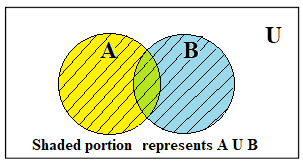
In the previous articles, we have studied the basics of set theory and its terminology. In this article, we shall study operations of sets including Union of sets, Intersection of sets, a complement of a set, Cartesian product of sets. The basic operations on sets are: Union of sets Intersection of sets A complement of […]
Introduction to Concept of Sets
A set is a collection of well-defined objects. These objects may be actually listed or may be specified by a rule. Sets are denoted by a capital letter like A, B, C…. and an object belonging to them (element of the set) are denoted by a small case letter like a, b, c,… of the […]
Properties of Substance

Science > Chemistry > Introduction to Chemistry > Properties of Substance All matter has physical and chemical properties. Extensive properties are those properties of a substance which depend on the amount of substance. They vary with the amount of the substance. Examples: Mass, weight, and volume. Intensive properties are those properties of a substance which do not depend […]