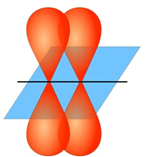
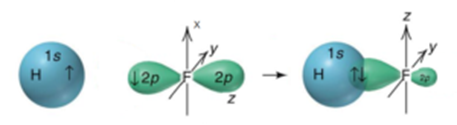
Science > Chemistry > Physical Chemistry > Nature of Chemical Bond > Hybridization of Orbitals In this article, we shall study the concept of hybridization of orbitals. This concept overcomes the limitations of valence bond theory. Need for Hybridization Concept: A simple approach based on the overlap of s and p orbitals can be applied […]
Categories
Hybridization of Orbitals
- Post author By Hemant More
- Post date March 31, 2020
- 1 Comment on Hybridization of Orbitals
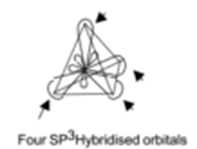
- Tags Axial overlap, Chemistry, Covalent bond, Excited state, Formation of Ammonia molecule, Formation of Methane molecule, Formation of water molecule, Geometry of molecule, Ground state, Hybridisation, Hybridization, Hybridized orbitals, Hybridized state, Lateral overlap, Nature of chemical bond, Overlapping of orbitals, P-P overlap, pi bond, S-P overlap, S-S overlap, sigma bond, SP hybridization, SP2 hybridization, SP3 hybridization, Tetrahedral geometry, Valence bond theory