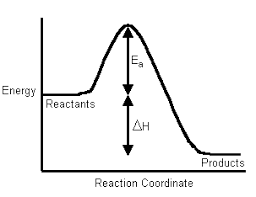
Science > Chemistry > Chemical Thermodynamics and Energetics > Bond Enthalpy In this article, we shall study the concept of bond enthalpy. During the formation of the bond, energy is evolved or released. Thus the bond formation reaction is exothermic. Due to the release of energy molecule formed is at a low energy level and […]
Bond Enthalpy