Science > Chemistry > Solutions and Their Colligative Properties > Short-cut Methods For Calculating Concentration of Solutions
In this article, we shall study short-cut methods to calculate molality, molarity, etc.
These methods can only be used in competitive exams only.
Direct Formulae to Calculate Molality and Molarity:
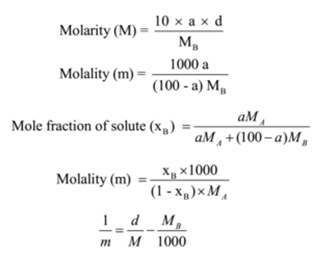
Where M = molarity in mol L-1 or M
m = molality in mol kg-1 or m
a = % by mass of solute
d = density of solution in g/mL or g cm-3.
MB = Molecular mass of solute in grams
MA = Molecular mass of solvent in grams
Note: When using these formulae, take care that the quantities are in prescribed units
Molecular masses of certain substances in grams:
Water H2O (18), Benzene C6H6 (78), Sodium hydroxide NaOH (40), Hydrogen chloride HCl (36.5), Sulphuric acid H2SO4 (98), potassium hydroxide KOH (56), Acetic acid (60), Sodium carbonate Na2CO3 (116),
Numerical Problems to Calculate Molality and Molarity:
Example – 01:
The density of a solution containing 13 % by mass of sulphuric acid is 1.09 g/mL. Calculate molarity and normality of the solution
Given: a = 13, d = 1.09 g/mL
To Find: Molarity (M) =? and Normality (N) =?
Solution:
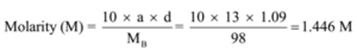
n = Molecular mass/equivalent mass = 98 g/49 g = 2
Normality = molarity x n = 1.446 x 2 = 2.892 N
Example – 02:
The density of 2.03 M solution of acetic acid (molecular mass = 60) in water is 1.017 g/mL. Calculate molality of solution
Given: M = 2.03, MB = 60 g mol-1, d = 1.017 g/mL
To Find: Molality (m) = ?
Solution:

molality = m = 1/0.4410 = 2.268 molal
Example – 03:
The density of 10.0% by mass of KCl solution in water is 1.06 g/mL. Calculate the molality, molarity and mole fraction of KCl.
Given: a = 10, d = 1.06 g/mL
To Find: Molarity (M) =?, molality (m) =?, mole fraction (XB) =?
Solution:

Ans: Molarity 1.42 M, Molality = 1.491 m, Mole fraction = 0.0261
Example – 04:
0.8 M solution of H2SO4 has a density of 1.06 g/cm3. calculate molality and mole fraction
Given: M = 0.8 M, d = 1.06 g/cm3.
To Find: Molality (m) =?, mole fraction (XB) =?
Solution:

molality = m = 1/1.227 = 0.814 molal

0.814 x 18 x (1 – XB) = 1000 XB
14.652 – 14.652 XB = 1000 XB
1014.652 XB = 14.652
XB = 14.652/1014.652 = 0.014
Example – 05:
A 6.90 M solution of KOH in water contains 30% by mass of KOH. Calculate the density of solution.
Given: M = 6.90 M, a = 30
To Find: density of solution = d = ?
Solution:

Ans: Density of solution = 1.288 g/mL
Example – 06:
An aqueous solution of NaOH is marked 10% (w/w). The density of the solution is 1.070 g cm-3. Calculate molality, molarity and mole fraction of NaOH in water. Given Na = 23, H =1 , O = 16
Given: a = 10, d = 1.070 g cm-3,
To Find: mole fraction =? molarity = ? and molality =?
Solution:

Example – 07:
Calculate the mole fraction of solute in its 2 molal aqueous solution.
Given: molality = 2 molal
To Find: Mole fraction =?
Solution:

Related Topics
Solutions and Their Colligative Properties
- Solutions and Their Types
- Solutions of Solids and Liquids
- Concentration of Solution
- Numerical Problems on Percentage by Mass and Volume
- Numerical Problems on Mole Fraction
- Numerical Problems on Molarity
- Numerical Problems on Molality
- Solutions of Gases in Liquid
- Ideal and Non-ideal Solutions
- Lowering of Vapour Pressure
- Numerical Problems on Lowering of Vapour Pressure
- Elevation in Boiling Point and Depression in Freezing Point
- Osmosis and Osmotic Pressure

3 replies on “Short-cut Methods For Calculating Concentration of Solutions”
NICE NO VERY NICE SHORTCUT FORMULAS
Much useful to me
Much educative