Science > Chemistry > Solutions and Their Colligative Properties > Boiling Point Elevation and Freezing Point Depression
In this article, we shall study two colligative properties of solutions, namely elevation of boiling point and depression in freezing point due to addition of solute.
Elevation of Boiling Point:
Boiling Point of a Liquid:
Boiling point is defined as the temperature at which the vapour pressure of the liquid becomes equal to the atmospheric pressure. The boiling point of a liquid is a characteristic property and can be treated as criteria for the purity of liquid. It increases with the increase in external pressure. Liquids having greater intermolecular forces have high boiling points.
Elevation of Boiling Point of a Liquid:
The vapour pressure of the solution of non-volatile solute is always less than the vapour pressure of the pure solvent.
At the boiling point of pure solvent, the solution will not boil because its vapour pressure of the solution is less than the vapour pressure of the pure solvent. Thus vapour pressure of the solution is less than the external pressure. To boil the solution we have to the increases vapour pressure of the solution to make it equal with external pressure. It is achieved by increasing the temperature of the solution. Thus there is an elevation of the boiling point of the liquid.
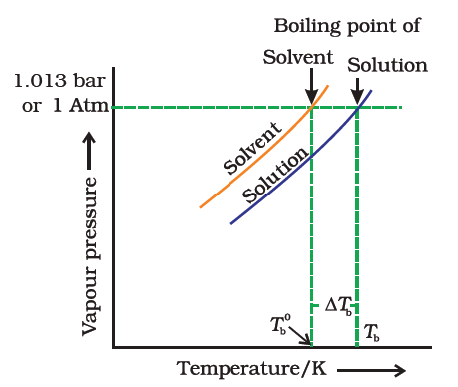
Let Tb0 be the boiling point of pure solvent and Tb be the boiling point of the solution. The increase in the boiling point Δ Tb = Tb – Tb0 is known as the elevation of boiling point. The elevation of boiling point (ΔTb) is directly proportional to the lowering of vapour pressure (Δp).
Thus Δ Tb α Δp.
Experiments have shown that for dilute solutions the elevation of boiling point ( Δ Tb ) is directly proportional to the molal concentration of the solute in a solution. Thus, the elevation of boiling point also depends on the number of solute molecules rather than their nature.
Δ Tb α m
Δ Tb = Kb m ………….. (1)
Here m (molality) is the number of moles of solute dissolved in 1 kg of solvent and the constant of proportionality, Kb is called Boiling Point Elevation Constant or Molal Elevation Constant (Ebullioscopic Constant). The unit of Kb is K kg mol-1.
The molal elevation of boiling point constant is defined as the elevation of boiling point produced when one mole of solute is dissolved in 1 kg of solvent.
Now
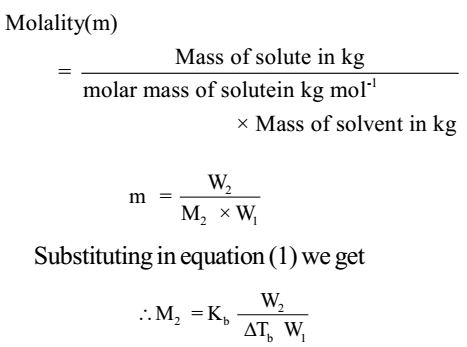
The experimental method to determine the molecular mass of non-volatile solute by determining boiling points of pure solvent and solution of known concentration is called ebullioscopy.
Depression of Freezing Point:
Freezing Point of a Liquid:
The freezing point of a liquid is a temperature at which the vapour pressure of solid is equal to the vapour pressure of the liquid.
Depression of Freezing Point of a Liquid:
The lowering of the vapour pressure of a solution causes a lowering of the freezing point compared to that of the pure solvent.
We know that at the freezing point of a substance, the solid phase is in dynamic equilibrium with the liquid phase. A solution will freeze when its vapour pressure equals the vapour pressure of the pure solid solvent as is clear from the graph.

According to Raoult’s law, when a non-volatile solid is added to the solvent its vapour pressure decreases and now it would become equal to that of solid solvent at a lower temperature. Thus, the freezing point of the solvent decreases.
Let Tf0 be the freezing point of pure solvent and Tf be the freezing point of the solution. The increase in the freezing point Δ Tf = Tf – Tf0 is known as depression of freezing point.
The depression of freezing point (Δ Tf) is directly proportional to the lowering of vapour pressure (Δp).
Thus, Δ Tf α Δp.
Experiments have shown that for dilute solutions the depression of freezing point (Δ Tf) is directly proportional to the molal concentration of the solute in a solution. Thus, the depression of freezing point also depends on the number of solute molecules rather than their nature.
Δ Tf α m
Δ Tf = Kf m ………….. (1)
Here m (molality) is the number of moles of solute dissolved in 1 kg of solvent and the constant of proportionality, Kf is called Freezing Point Elevation Constant or Molal Elevation Constant (cryoscopic Constant). The unit of Kf is K kg mol-1.
The molal elevation of freezing point constant is defined as the depression of freezing point produced when one mole of solute is dissolved in 1 kg of solvent.
Now,

The experimental method to determine the molecular mass of non-volatile solute by determining freezing points of pure solvent and solution of known concentration is called cryoscopy.
Problems:
- Which of the following aqueous solutions will have maximum depression in freezing point. a) 0.5 M Li2SO4 b) 1 M NaCl c) 0.5 M Al2(SO4)3 d) 0.5 M BaCl2
- A solution containing 0.73 g of camphor (molar mass 152 g mol-1) in 36.8 g of acetone (boiling point 56.3° C) boils at 56.55° C. A solution of 0.564 g of unknown compound in the same weight of acetone boils at 56.46° C. calculate molar mass of the unknown compound.
- 1.0 x 10-3 kg of urea when dissolved in 0.0985 kg of a solvent, decreases the freezing point of the solvent by 0.211 K. 1.6 x 10-3 kg of another non-electrolyte solute when dissolved in 0.086 kg of the same solvent depresses the freezing point by 0.34 K. Calculate the molar mass of another solute.
- Which of the following aqueous solutions will have minimum elevation in boiling point. a) 0.1 M KCl b) 0.05 M NaCl c) 1 M AlPO4 d) 0.1 M MgSO4
- The boiling point of a solvent is 80.2° C. When 0.419 g of the solute of molar mass 252.4 g mol-1, is dissolved in 75 g of above solvent, the boiling point of the solution is found to be 80.26° C. Find molal elevation constant.
Related Topics
Solutions and Their Colligative Properties
- Solutions and Their Types
- Solutions of Solids and Liquids
- Concentration of Solution
- Numerical Problems on Percentage by Mass and Volume
- Numerical Problems on Mole Fraction
- Numerical Problems on Molarity
- Numerical Problems on Molality
- Short Cuts For Above Numerical Problems
- Solutions of Gases in Liquid
- Ideal and Non-ideal Solutions
- Lowering of Vapour Pressure
- Numerical Problems on Lowering of Vapour Pressure
- Osmosis and Osmotic Pressure