Science > Chemistry > Solutions and Their Colligative Properties > Numerical Problems on Lowering of Vapour Pressure
In this article, we shall study to solve problems based on relative lowering of vapour pressure and to calculate the molecular mass of a solute.
Example – 01:
The vapour pressure of a pure liquid at 298K is 4 x 104 N/m2. When a non-volatile solute is dissolved the vapour pressure becomes 3.65 x 104 N/m2. Calculate relative vapour pressure, lowering of vapour pressure and relative lowering of vapour pressure
Given: Vapour pressure of pure liquid = po = 4 x 104 N/m2, vapour pressure of solution = p = 3.65 x 104 N/m2, temperature = T = 298 K,
To Find: Relative vapour pressure = p/po =? Lowering of vapour pressure = po – p =? and relative lowering of pressure = (po – p)/po = ?
Solution:
Relative vapour pressure = p/po = (3.65 x 104 N/m2)/(4 x 104 N/m2) = 0.9125
Lowering of vapour pressure = po – p = 4 x 104 N/m2 – 3.65 x 104 N/m2
Lowering of vapour pressure = 0.35 x 104 N/m2 = 3.5 x 103 N/m2
Relative lowering of pressure = (po – p)/po = (3.5 x 103 N/m2)/(4 x 104 N/m2) = 0.0875
Ans: Relative vapour pressure = 0.9125, Lowering of vapour pressure = 3.5 x 103 N/m2, Relative lowering of pressure = 0.0875
Example – 02:
The vapour pressure of a solution containing 13 × 10-3 kg of solute in 0.1 kg of water at 298 K is 27.371 mm Hg. calculate the molar mass of the solute. Given that the vapour pressure of water at 298 K is 28.065 mm Hg.
Given: mass of solute W2 = 13 × 10-3 kg, mass of solvent (water) = W1 = 0.1 kg, vapour pressure of pure solvent (water) = po = 28.065 mm of Hg, vapour pressure of solution = p = 27.371 mm of Hg, temperature = T = 298 K, Molecular mass of solvent (water) = M1 = 18 g mol-1
To Find: Molecular mass of solute = M2 =?
Solution:
For dilute solution relative lowering of vapour pressure is given by

Ans: Molecular mass of solute is 94.63 g mol-1
Example – 03:
The vapour pressure of pure benzene at a certain temperature is 640 mm Hg. A non-volatile solute of a mass 2.175 × 10-3 kg is added to 39.0 × 10-3 kg of benzene. The vapour pressure of a solution is 600 mm Hg. What is the molar mass of the solute? Given C= 12, H = 1.
Given: mass of solute W2 = 2.175 × 10-3 kg, mass of solvent = W1 = 39.0 × 10-3 kg, vapour pressure of pure solvent (benzene) = po = 640 mm of Hg, vapour pressure of solution = p = 600 mm of Hg,
To Find: Molecular mass of solute = M2 =?
Solution:
Molecular mass of solvent (benzene C6H6) = M1 = 12 x 6 + 1 x 6 = 78 g mol-1
For dilute solution relative lowering of vapour pressure is given by
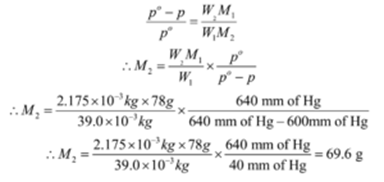
Ans: Molecular mass of solute is 69.6 g mol-1
Example – 04:
In an experiment, 18.04 g of mannitol were dissolved in 100 g of water. The vapour pressure of water was lowered by 0.309 mm Hg from 17.535 mm Hg. Calculate the molar mass of mannitol.
Given: mass of solute (mannitol) W2 = 18.04 g, mass of solvent (water) = W1 = 100g, vapour pressure of pure solvent (water) = po = 17.535 mm of Hg, decrease in vapour pressure of solution = po – p = 0.309 mm of Hg, Molecular mass of solvent (water) = M1 = 18 g mol-1
To Find: Molecular mass of solute (mannitol) = M2 =?
Solution:
For dilute solution relative lowering of vapour pressure is given by

Ans: Molecular mass of solute is 184.3 g mol-1.
Example – 05:
A solution is prepared from 26.2 × 10-3 kg of an unknown substance and 112.0 × 10-3 kg acetone at 313 K. The vapour pressure of pure acetone at this temperature is 0.526 atm. Calculate the vapour pressure of solution if the molar mass of a substance is 273.52 × 10-3 kg mol-1. Given C = 12, H = 1, O = 16.
Given: mass of solute W2 = 26.2 × 10-3 kg, mass of solvent = W1 = 112.0 × 10-3 kg, vapour pressure of pure solvent (acetone = po = 0.526 atm, Molecular mass of solute = M2 = 273.52 × 10-3 kg
To Find: vapour pressure of solution = p =?
Solution:
Molecular mass of solvent (acetone (CH3)2CO) = M1 = 12 x 3 + 1 x 6 + 16 x 1
Molecular mass of solvent (acetone (CH3)2CO) = M1 = 58 g mol-1 = 58 × 10-3 kg
For dilute solution relative lowering of vapour pressure is given by

Ans: Vapour pressure of solution is 0.500 atm.
Example – 06:
The vapour pressure of water at 20 °C is 17 mm Hg. Calculate the vapour pressure of a solution containing 2.8 g of urea (NH2CONH2) in 50 g of water.
Given: Temperature of water = 20 °C = 20 + 273 = 293 K, vapour pressure of pure solvent (water) = po = 17 mm of Hg, mass of urea (solute)(NH2CONH2) = W2 = 2.8 g, mass of water (solvent) = W2 = 50 g
To Find: Vapour pressure of solution = p =?
Solution:
The molecular mass of solute urea (NH2CONH2) = M2
M2 = 14 g x 2 + 1 g x 4 + 12 g x 1 + 16 g x 1 = 60 g mol-1.
The molecular mass of water M1 = 18 g mol-1.

Ans: Vapour pressure of solution = 16.714 mm of Hg.
Example – 07:
At 300 K the vapour pressure of water is 1.2 x 104 Pa. 0.8 x 10-2 kg of oxalic acid (molecular mass = 126) is dissolved in 700 cm3 of water at the same temperature, find the vapour pressure of the solution.
Given: Temperature of water = 300 K, vapour pressure of pure solvent (water) = po = 1.2 x 104 Pa, mass of solute (oxalic acid) = W2 = 0.8 x 10-2 kg, volume of water (solvent) = 700 cm3, molecular mass of water = M1 = 18 g mol-1, molecular mass of solute (oxalic acid) = M2 = 126 g mol-1.
To Find: Vapour pressure of solution = p =?
Solution:
Volume of water = 700 cm3
Mass of water = W1 = 700 cm3 x 1 g cm-3 = 700 g = 0.7 kg

Ans: Vapour pressure of solution = 1.198 x 104 Pa.
Example – 08:
Calculate the decrease in the vapour pressure when 1.81 g x 10-2 kg of a solute (molecular mass = 57) is dissolved in 0.1 kg of water. Vapour pressure of water is 1.223 x 104 Pa.
Given: Vapour pressure of pure solvent (water) = po = 1.223 x 104 Pa, mass of solute = W2 = 1.81 x 10-2 kg, mass of water (solvent) = 0.1 kg, molecular mass of water = M1 = 18 g mol-1, molecular mass of solute = M2 = 57 g mol-1.
To Find: Decrease in vapour pressure of solution = po – p =?
Solution:

Ans: Decrease in vapour pressure is 699 Pa
Example – 09:
A solution containing 30 g of a non-volatile solute in exactly 90 g of water has a vapour pressure of 21.85 mm of Hg at 25 oC. Further 18 g of water is then added to the solution. The new vapour pressure becomes 22.15 mm of Hg at 25 oC. Calculate the molecular mass of the solute and the vapour pressure of water at 25 oC.
Given: Temperature of water = 25 oC = 25 + 273 = 298 K
For solution 1: Mass of solute = W2 = 30 g, mass of solvent = W1= 90 g, solution vapour pressure = p = 21.85 mm of Hg
For solution 2: Mass of solute = W2 = 30 g, mass of solvent = W1= 90 g + 18 g = 108 g, solution vapour pressure = p = 22.15 mm of Hg
To Find: Molecular mass of solute = M2 =? vapour pressure of pure water solution = po =?
Solution:

5po – 109.25 = 6po – 132.9
po = 132.9 -109.25 = 23.65 mm of Hg
Substituting in equation (1)

Ans: Molecular mass of solute is 72.83 u and vapour pressure of pure water solution 23.65 mm
Related Topics
Solutions and Their Colligative Properties
- Solutions and Their Types
- Solutions of Solids and Liquids
- Concentration of Solution
- Numerical Problems on Percentage by Mass and Volume
- Numerical Problems on Mole Fraction
- Numerical Problems on Molarity
- Numerical Problems on Molality
- Short Cuts For Above Numerical Problems
- Solutions of Gases in Liquid
- Ideal and Non-ideal Solutions
- Lowering of Vapour Pressure
- Elevation in Boiling Point and Depression in Freezing Point
- Osmosis and Osmotic Pressure
5 replies on “Numerical Problems on Lowering of Vapour Pressure”
Really it very useful to us and it is have nice problems
Wow that’s a very nice problems,of course I have learned a lot.
God bless you sir
It is really understandable
Very good.. and useful a lot for exam.
Good questions to clear the basics.
Thank u very much