Science > Chemistry > Solutions and Their Colligative Properties > Percentage Composition
In this article, we shall learn to calculate percentages by mass and percentage by volume of a solution.
Problems on Percentage by Mass
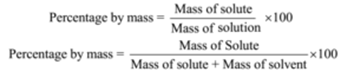
Example – 01:
6 g of urea was dissolved in 500 g of water. Calculate the percentage by mass of urea in the solution.
Given: Mass of solute (urea) = 6 g, Mass of solvent (water) = 500 g
To Find: Percent by mass =?
Solution:
Mass of solution = Mass of solute + Mass of solvent = 6 g + 500 g = 506 g
Percentage by mass of urea = (Mass of solute/Mass of solution) x 100
= (6/506) x 100 = 1.186%
Example – 02:
34.2 g of glucose is dissolved in 400 g of water. Calculate the percentage by mass of glucose solution.
Given: Mass of solute (glucose) = 34.2 g, Mass of solvent (water) = 400 g
To Find: Percentage by mass =?
Solution:
Mass of solution = Mass of solute + Mass of solvent = 34.2 g + 400 g = 434.2 g
Percentage by mass = (Mass of solute/Mass of solution) x 100
= (34.2/434.2) x 100 = 7.877%
Example – 03:
A solution is prepared by dissolving 15 g of cane sugar in 60 g of water. Calculate the mass percent of each component of the solution.
Given: Mass of solute (cane sugar) = 15 g, Mass of solvent (water) = 60 g
To Find: Mass percent of cane sugar and water =?
Solution:
Mass of solution = mass of solute + mass of solvent = 15 g + 60 g = 75 g
Percentage by mass of solute c(cane sugar) = (Mass of solute/Mass of solution) x 100
Mass percent of solute (cane sugar) = (15 g/75 g) x 100 = 20%
Mass percent of solvent (water) = 100 – 20 = 80%
Example – 04:
Calculate the mass percentage of benzene and carbon tetrachloride if 22 g of benzene is dissolved in 122 g of carbon tetrachloride.
Given: Mass of solute (benzene) = 22 g, Mass of solvent (carbon tetrachloride) = 122 g.
To Find: Mass percentage of benzene and carbon tetrachloride.
Solution:
Mass of solution = mass of solute + mass of solvent
Mass of solution = 22 g + 122 g = 144 g
Percentage by mass = (Mass of solute/Mass of solution) x 100
Percentage of benzene by mass = (22 g/144 g) x 100 = 15.28%
Percentage of carbon tetrachloride by mass = 100 – 15.28 = 84.72%
Example – 05:
A solution is prepared by dissolving a certain amount of solute in 500 g of water. The percentage by mass of a solute in a solution is 2.38. Calculate the mass of solute
Given: Mass of solvent = 500 g, percentage by mass = 2.38
To Find: Mass of solute =?
Solution:
Let the mass of solute = x g
Mass of solution = Mass of solute + Mass of solvent = x g + 500 g = (x + 500) g
Percentage by mass = (Mass of solute/Mass of solution) x 100
2.38 = (x g/(x + 500) g) x 100
2.38 (x + 500) = 100x
2.38x + 1190 = 100x
1190 = 97.62 x
x = 1190/97.62 = 12.19 g
The mass of solute is 12.19 g
Example – 06:
Calculate the masses of cane sugar and water required to prepare 250 g of 25% cane sugar solution.
Given: 250 g of 25% cane sugar solution
To Find: Masses of cane sugar and water =?
Solution:
Let the mass of cane sugar = x g
Mass of solution = 250 g
Percentage by mass = (Mass of solute/Mass of solution) x 100
25 = (x g/250 g) x 100
25 x 250 g = 100x
6250 g = 100x
x = 6250 g/100 = 62.5 g
Mass of cane sugar = 62.5 g
Mass of water = 250 g – 62.5 g = 187.5 g
Example – 07:
15 g of methyl alcohol is present in 100 mL of solution. If the density of solution is 0.96 g mL-1. Calculate the mass percentage of methyl alcohol solution.
Given: Mass of solute (methyl alcohol) = 15 g, Volume of solution = V = 100 mL, Density of solution = d = 0.96 g mL-1.
To Find: mass percentage of methyl alcohol =?
Solution:
Mass of solution = volume x density = 100 mL x 0.96 g mL-1 = 96 g
Mass percentage = (Mass of solute/Mass of solution) x 100
Mass percentage of benzene = (15 g/96 g) x 100 = 15.625%
Example – 08:
The density of the solution of salt X is 1.15 g mL-1. 20 mL of the solution when completely evaporated gave a residue of 4.6 g of the salt. Calculate the mass percentage of solute in the solution.
Given: Volume of solution = V = 20 mL, density of solution = d = 1.15 g mL-1, Mass of solute = 4.6 g
To Find: mass percentage of solute in the solution =?
Solution:
Mass of solution = volume x density = 20 mL x 1.15 g mL-1 = 23 g
Percentage by mass = (Mass of solute/Mass of solution) x 100
Percentage of solute by mass = (4.6 g/23 g) x 100 = 20%
Example – 09:
40% by mass of urea is obtained when 190 g of urea is dissolved in 400 mL of water. Calculate the density of solution.
Given: % by mass of urea solution = 40%, mass of solvent (water) = 400 mL.
To Find: Density of solution =?
Solution:
Percentage by mass = (Mass of solute/Mass of solution) x 100
Mass of solution = (Mass of solute/Percentage by mass) x 100
Mass of solution = (190 g/40) x 100 = 475 g
The volume of solvent (water) = 400 mL = Volume of solution
Density of solution = mass of solution /volume of solution
Density of solution = (475 g) / (400 mL) = 1.19 g mL-1
Example – 10:
Calculate percent composition in terms of mass of a solution obtained by mixing 300 g of 25% solution of NH4NO3 with 400 g of a 40% solution of solute X.
Given: 300 g of 25% solution of NH4NO3 mixed with 400 g of a 40% solution of solute X
To Find: percentage composition in terms of mass =?
Solution:
Consider 300 g of 25% solution of NH4NO3
Mass of solute in this solution = 25% of 300 g = (25/100) x 300 g = 75 g
Consider 400 g of a 40% solution of solute X
Mass of solute in this solution = 40% of 400 g = (40/100) x 400 g = 160 g
Now let us consider the solution obtained by mixing
Total mass of solute = WB = 75 g + 160 g = 235 g
Total mass of solution = WA = 300 g + 400 g = 700 g
Percentage by mass = (Mass of solute/Mass of solution) x 100
Percentage of solute by mass = (235 g/700 g) x 100 = 33.57%
Percentage of solvent by mass = 100 – 33.57 = 66.43%
Example – 11:
Calculate percentage composition in terms of mass of a solution obtained by mixing 100 g of 30% solution of NaOH with 150 g of a 40% solution of NaOH.
Given: 100 g of 30% solution of NaOH mixed with 150 g of a 40% solution of NaOH
To Find: percentage composition in terms of mass =?
Solution:
Consider 100 g of 30% solution of NaOH
Mass of solute in this solution = 30% of 100 g = (30/100) x 100 g = 30 g
Consider 150 g of a 40% solution of NaOH
Mass of solute in this solution = 40% of 150 g = (40/100) x 150 g = 60 g
Now let us consider the solution obtained by mixing
Total mass of solute = WB = 30 g + 60 g = 90 g
Total mass of solution = WA = 100 g + 150 g = 250 g
Percentage by mass = (Mass of solute/Mass of solution) x 100
Percentage of solute by mass = (90 g/250 g) x 100 = 36%
Percentage of solvent by mass = 100 – 36 = 64%
Problems on Percentage by Volume:

Example – 12:
12.8 cm3 of benzene is dissolved in 16.8 cm3 of xylene. Calculate percentage by volume of benzene.
Given: Volume of solute = 12.8 cm3, Volume of solvent = 16.8 cm3
To Find: Percentage by volume =?
Solution:
Volume of solution = Volume of solute + Volume of solvent
Volume of solution = 12.8 cm3+ 16.8 cm3 = 29.6 cm3
Percentage by volume = (Volume of solute/Volume of solution) x 100
Percentage of benzene by volume = (12.8 cm3/29.6 cm3) x 100 = 43.24 %
Example – 13:
58 cm3 of ethyl alcohol was dissolved in 400 cm3 of water to form 454 cm3 of a solution of ethyl alcohol. Calculate percentage by volume of ethyl alcohol in water. (12.78 % by volume)
Given: Volume of solute = 58 cm3, Volume of solution = 454 cm3
To Find: Percentage by volume =?
Solution:
Percentage by volume = (Volume of solute/Volume of solution) x 100
Percentage of ethyl alcohol by volume = (58 cm3/454 cm3) x 100 = 12.78%
Related Topics
Solutions and Their Colligative Properties
- Solutions and Their Types
- Solutions of Solids and Liquids
- Concentration of Solution
- Numerical Problems on Mole Fraction
- Numerical Problems on Molarity
- Numerical Problems on Molality
- Short Cuts For Above Numerical Problems
- Solutions of Gases in Liquid
- Ideal and Non-ideal Solutions
- Lowering of Vapour Pressure
- Numerical Problems on Lowering of Vapour Pressure
- Elevation in Boiling Point and Depression in Freezing Point
- Osmosis and Osmotic Pressure

7 replies on “Numerical Problems on Percentage by Mass”
Thankyou 💜
Thanks a lot
correction in question no 5 amount of solvent is 500g and in given its written wrong its 400 g
Thank you Himanshu for correction
Thank u it helped a lot
All numerical were like HOW WONDERFUL
thank you……so much