Science > Chemistry > Solutions and Their Colligative Properties > Solid and Liquid Solutions
In this article, we shall study solid and liquid solutions and the concept of solubility curves.
A solution of Solids in Liquids:
The dissolution of any substance in a liquid to form a solution is governed by the basic principle that the solute-solvent interaction is either similar to or greater than solute-solute and solvent-solvent interaction. Let us consider the dissolution of an ionic solid in water. There is an interionic attraction between the ions of solid. In this case, the cations and anions of the solid get attracted by the opposite ends of water dipoles.
If the ion-dipole interaction forces are stronger than interionic attraction, the ions are pulled out of crystal lattice and they pass into solution. Formed ions are called hydrated ions.
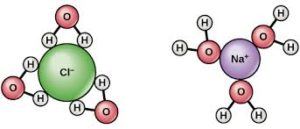
Molecular solids dissolve in the water on account of their capacity to form hydrogen bonds with water molecules.
The energy required to dismantle 1 mol of the crystal lattice is called lattice energy (ΔLH). The energy released during hydration is called hydration energy (ΔHydroH). Mathematically,
ΔSolH = ΔLH + ΔHydroH.
- Case – I: ΔHydroH ≥ ΔLH:Dissolution occurs and ΔSolH < 0
- Case – II: ΔHydroH marginally less than ΔLH: Dissolution occurs and ΔSolH > 0
- Case – III: ΔLH too large to compensate ΔHydroH: Dissolution does not occur.
Factors Affecting Solubility of a Solids in Liquids:
- Nature of solutes: Polar solvent (e.g. water) dissolves polar solute (e.g. NaCl, KCl) and non-polar solvents (CCl4, CS2) dissolve non-polar solutes (e.g. I2, S8).
- Temperature: The solubility of a solid in a liquid is significantly affected by temperature changes. Consider the equilibrium of solute particles dissolving and crystallizing.
Solute + Solvent ⇌ Solution
It is a dynamic equilibrium and follows Le Chatelier,s Principle. If in a nearly saturated solution, the dissolution process is endothermic (ΔsolH > 0), the solubility should increase with the rise in temperature if it is exothermic (ΔsolH < 0) the solubility should decrease.
- Pressure: Pressure does not have any significant effect on so the ability of solids in liquids. It is so because solids and liquids are highly incompressible and practically remain unaffected by changes in pressure.
A solution of Solids in Solids:
A solid solution of two or more metals or metals or metals with one or more non-metal is called an alloy.
Types of Solid Solutions:
- Substitutional: Solute
atoms occupy the regular lattice sites of the parent metal (solvent).
Substitutional solid solutions can be random (Cu-Ni) or ordered (Cu-Au). - Interstitial: Solute
atoms occupy the interstitial positions (Steel – C solute atoms in Fe).
Examples of Alloys:
| Alloy | Composition | Uses |
| Duralumin | Aluminum, copper, manganese, magnesium | aircraft, boats, railroad cars, and machinery because of its high strength and resistance to corrosion |
| Aluminium bronze | Aluminum, copper, manganese | heavy duty sleeve bearings, and machine tool ways. Aluminium bronze castings have exceptional corrosion resistance, high strength, toughness and wear resistance and good casting and welding characteristics. |
| Hardened Lead | 10 to 20 % antimony | bearings, bullets and shrapnel |
| Stainless Steel | Iron, chromium, nickel | Cutlery, surgical instruments, utensils |
| Spiegeleisen | Iron and 5 to 20 % manganese | Very hard hence used for making rails, safes and heavy machinery |
| Ferromanganeous | Iron and 70 to 80 % manganese | Very hard hence used for making rails, safes and heavy machinery |
| Manganin | 84 % Cu, 12 % Mn, 4% Ni | electrical measurement instrument due to zero temperature coefficient of resistance. |
Amalgams:
Alloys of mercury with other metals are called amalgams. This property of mercury is used for extraction of metals like silver and gold from their ores. These metals are further recovered by distillation of mercury.
A solution of Liquids in Liquids:
Evolution or Absorption of Heat During Formation of Solution:
When one liquid is dissolved in another, two processes should take place simultaneously. In the first, the solvent molecules move apart to accommodate the solute molecules while in the second, the solute molecules separate to get accommodated in the space provided by the solvent. For this process energy and absorbed. At the end of the formation of the solution, the solute and solvent molecules are brought together. This results in the release of energy. The net of the two energies gives the enthalpy of solution.
If there is a strong attraction between the solute and the solvent molecules, the heat released is more than the heat absorbed and more energy is released in the final step. Thus with the concept of energy, there are thee possibilities.
- Case – I: There is the absorption of heat (e.g. formation of a
solution of ethyl alcohol and water). - Case – II: There is an evolution of heat (e.g. formation of a solution
of acetone and water) - Case – III: There is neither absorption nor evolution of heat (e.g.
formation of a solution of benzene and carbon tetrachloride)
Effect of Relative Solubility of Solute in Solution:
When two liquids are mixed, there are three possibilities.
- Case – I: The two components are almost immiscible. It happens when
one liquid is polar and other in non-polar. e.g. a solution of water and
benzene, a solution of carbon tetrachloride and water, a solution of
benzene and alcohol. - Case – II: The miscibility of the component may be partial. This
happens when the intermolecular attraction of one liquid is different from the
intermolecular attraction of other liquid. e.g. a solution of water and
ether, a solution of phenol and water, a solution of nicotine and
water. - Case – III: The two components may be completely miscible. It happens
when the two liquids have the same nature. Either both are polar (e.g. a
solution of alcohol and water) or both are non-polar (e.g. a solution of
benzene and hexane, a solution of benzene and toluene).
Characteristics of miscible liquids:
- They are chemically alike.
- Both have the same nature.
- Dipole-dipole interaction is stronger.
- Molecular sizes of liquids are approximately the same.
Solubility Curves:
Solubility is measured by determining the maximum mass of a solute that can be dissolved in 100 g of a solvent at a given temperature. A solubility curve is a graph giving the relationship between the temperature and solubility of a particular solute. Thus the solubility curves give the idea about what mass of solute will dissolve in 100g (or 100mL) of water over a range of temperatures.
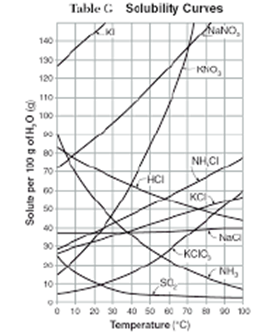
Solutes whose solubility curves move upward with increased temperature are typically solids because the solubility of solids increases with increased temperature. The steeper the incline of a solute, the more soluble the solute is because it doesn’t take as much of a temperature increase to dissolve the substance. This also means that more of the solute can be dissolved versus another substance at the same temperature. From the solubility curve, we can see that the solubility of KNO3 rises sharply with the increase in temperature. In solutions involving liquids and solids typically more solute can be dissolved at higher temperatures.
Solutes whose solubility curves move downward with increased temperature are typically gases because the solubility of gases decreases with increased temperature.
The flatter the line, the less soluble the solute it, because it takes a larger temperature in order for a solute to dissolve compared to a
steeper inclined substance. The solubility of NaCl almost remains constant.
Related Topics
Solutions and Their Colligative Properties
- Solutions and Their Types
- Concentration of Solution
- Numerical Problems on Percentage by Mass and Volume
- Numerical Problems on Mole Fraction
- Numerical Problems on Molarity
- Numerical Problems on Molality
- Short Cuts For Above Numerical Problems
- Solutions of Gases in Liquid
- Ideal and Non-ideal Solutions
- Lowering of Vapour Pressure
- Numerical Problems on Lowering of Vapour Pressure
- Elevation in Boiling Point and Depression in Freezing Point
- Osmosis and Osmotic Pressure
