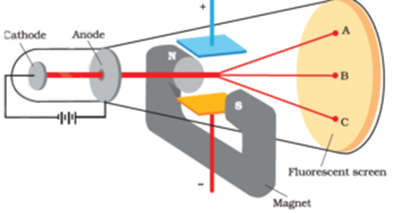
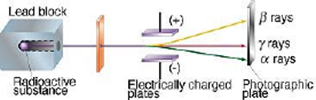
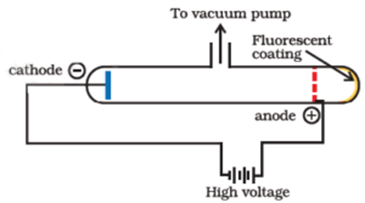
Science > Chemistry > Atomic Structure > Discovery of Proton and Neutron In last article, we have discussed, the discovery of electron and characteristics of electron. In this article, we shall discuss the discovery of proton and neutron and their characteristics. Discovery of Proton: Proton was discovered by E. Goldstein in 1886. He performed the […]
Categories
Discovery of Proton and Neutron
- Post author By Hemant More
- Post date July 30, 2020
- No Comments on Discovery of Proton and Neutron
- Tags Alpha particles, Atomic mass number, Atomic number, Atomic structure, Beta particles, canal rays, Cathode rays, Charge on electron, Charge to mass ratio, Chemistry, Dalton's atomic theory, Discovery of electron, Discovery of neutron, Discovery of proton, gamma radiations, gamma rays, Isobars, Isotones, Isotopes, Mass on electron, Millikan's oil drop experiment, Mosley's contribution, Natural radioactivity, Neutron number, Neutrons, Protons, Radioactivity, Rutherford's model of atom, Structure of atom, Thomson's experiment, Thomson's model of atom, X-Rays