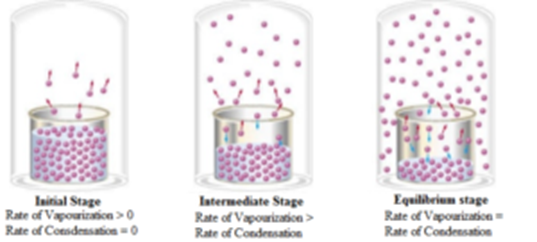
Science > Chemistry > Chemical Equilibrium > Writing Expression for Equilibrium Constant In this article, we shall stuudy to write expression for equilibrium constant. Steps Involved in Writing Expression for Equilibrium Constant of a Reaction: Write the balanced chemical equation for the reaction. Write the products of equilibrium concentrations of the products in the numerator. […]
Categories
Writing Expression for Equilibrium Constant
- Post author By Hemant More
- Post date April 2, 2020
- No Comments on Writing Expression for Equilibrium Constant
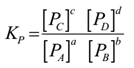
- Tags Active mass, Chemical equilibrium, Chemical reaction, Chemistry, Concentration quotient, Dynamic equilibrium, Endothermic reaction, Equilibrium, Equilibrium concentration, Equilibrium constant., Equilibrium mixture, Exothermic reaction, Heterogeneous equilibrium, Heterogeneous reaction, Homogeneous equilibrium, Homogeneous reaction, Irreversible reaction, Law of chemical equilibrium, Law of mass action, Partial pressure, Physical equilibrium, Products, Rate of chemical reaction, Reactants, Reversible reaction