Science > Chemistry > Atomic Structure > Problems on Calculation of Number of Electrons, Protons, and Neutrons In this article, we shall study to solve problems on the calculations of the number of electrons, protons, and neutrons in atoms, molecules, and species. Example 01: Calculate the charge and mass of 1 mole of electrons. Solution: […]
Tag: Atomic structure
Science > Chemistry > Atomic Structure > Problems Based on Atomic Number, Mass Number, and Neutron Number In this article, we shall study to solve problems based on the calculation of atomic number, atomic mass number, and neutron number Atomic number (Z) : The number of protons (positive charge) present in the nucleus of an […]
Dual Nature of Radiations
Science > Chemistry > Atomic Structure > Dual Nature of Radiations Light and other electromagnetic radiations have dual nature viz: the particle nature and the wave nature. Wave Nature of Radiations: Radiation is the form of energy, which can be transferred from one point to another point in space. Radiations are considered to be transmitted by wave […]
Science > Chemistry > Atomic Structure > Heisenberg’s Uncertainty Principle In this article, we shall study Heisenberg’s uncertainty principle, the concept of quantum numbers, and the model of an atom based on the quantum numbers. Heisenberg’s Uncertainty Principle: Werner Heisenberg a German physicist in 1927, stated the uncertainty principle which is the consequence of dual behaviour of […]
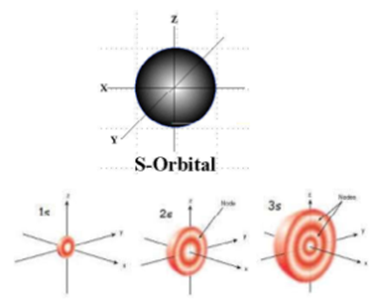
Science > Chemistry > Atomic Structure > Quantum Numbers and Quantum Model of an Atom In this article, we shall study the concept of quantum numbers and the model of an atom based on the quantum numbers. Concept of Quantum Numbers: In 1926, Erwin Schrodinger put forward a theory of atom called as a quantum […]
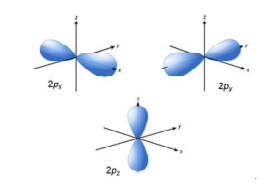
Science > Chemistry > Atomic Structure > Quantum Numbers and Shapes of Orbitals In this article, we shall study the concept of quantum numbers and different types of orbitals and their shapes. Difference Between Shell, Subshell, and Orbital: All electrons that have the same value for n (the principal quantum number) are in the same […]
Electronic Configuration
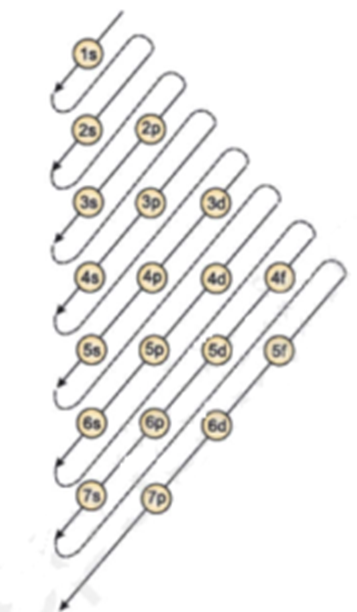
Science > Chemistry > Atomic Structure > Electronic Configuration The distribution of electrons in various shells and orbitals of an atom is known as electronic configuration. Generally electronic configuration is given by nlx. Where n is shell number, l is subshell and x are the number of electrons. Aufbau Principle: The arrangement of the electrons […]
Rutherford’s Atomic Model

Science > Chemistry > Atomic Structure > Rutherford’s Atomic Model In this article, we shall study Thomson’s model of an atom and its deficiencies and Rutherford’s atomic model and its deficiencies. Thomson’s Model of an Atom: J. J. Thomson, in 1898, proposed that an atom possesses a spherical shape (radius approximately 10–10 m) in which the positive charge […]

Science > Chemistry > Atomic Structure > Discovery of Proton and Neutron In last article, we have discussed, the discovery of electron and characteristics of electron. In this article, we shall discuss the discovery of proton and neutron and their characteristics. Discovery of Proton: Proton was discovered by E. Goldstein in 1886. He performed the […]
Discovery of Electron

Science > Chemistry > Atomic Structure > Discovery of Electron In this article, we shall study earlier concepts of an atom, discovery of electron, and its characteristics. 600 B.C. Indian saint and philosopher Maharshi Kanad proposed that matter is made up of the smallest individual particles. He called these particles ‘paramanu’. Around 400 B.C. The […]