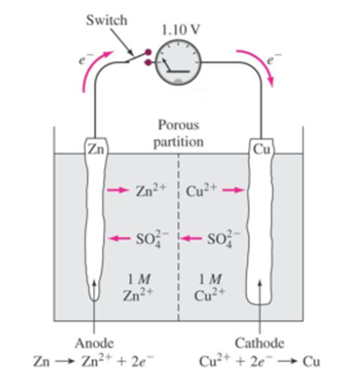
Science > Chemistry > Electrochemistry > Primary Electrochemical Cells Types of Electrochemical Cell: An electrochemical cell is a device which is used to generate electrical energy at the expense of spontaneous oxidation-reduction reaction. An electrochemical cell is also known as a galvanic cell or a voltaic cell. In an electrochemical cell, chemical energy is converted into […]
Categories
Primary Electrochemical Cells
- Post author By Hemant More
- Post date December 23, 2019
- No Comments on Primary Electrochemical Cells
- Tags Anion, Anode, Button cell, Cathode, Cation, Cell reaction, Construction of cell, Coulomb, Daniel cell, Dry Cell, Electrochemical cell, Electrode, Electrolyte, Electrolytic cell, emff of cell, Fuel cell, Galvanic cell, Lead accumulator, Leclanche cell, Non-electrolyte, Oxidation, Primary cell, Redox reaction, Reduction, Reversibility of cell, Secondary cell, Voltaic cell, watt, Working of cell