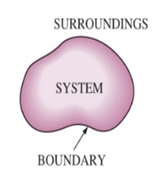
Science > Chemistry > Chemical Thermodynamics and Energetics > Introduction Chemical Thermodynamics and its Scope: Energy stored in chemical substances is called chemical energy. Thermodynamics is the branch of science that deals with the different forms of energy, the quantitative relationships between them and the energy changes that occur in the physical and chemical process. […]