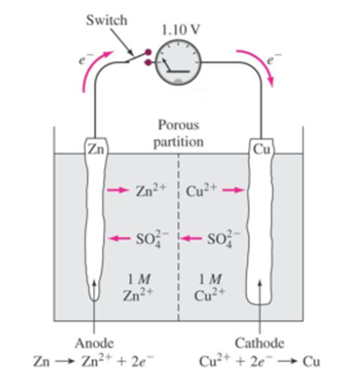
Science > Chemistry > Electrochemistry > Nernst Theory In this article, we shall study the Nernst theory of electrode potential, Nernst equation, and its use. Single Electrode or Half cell or Electrode Couple: A single electrode or half cell or electrode couple is produced when a metal is dipped in the solution of its own […]
Categories
Concept of Electrode Potential: Nernst Theory
- Post author By Hemant More
- Post date December 24, 2019
- No Comments on Concept of Electrode Potential: Nernst Theory
- Tags Anion, Anode, Button cell, Calomel electrode, Cathode, Cation, Cell reaction, Charging of cell, Chlorine gas electrode, Construction of cell, Convention of representation of cell, Coulomb, Daniel cell, De-electronation, Discharging of cell, Dry Cell, Electrochemical cell, Electrode, Electrode couple, Electrolyte, Electrolytic cell, Electronation, emff of cell, Fuel cell, Galvanic cell, Gas electrode, Indicator electrode, Lead accumulator, Leclanche cell, Maintenance of lead accumulator, Metal - metal ion electrode, Metal-Sparingly Soluble Metal Salt Electrode, Nernst equation, Nernst theory, Nickel cadmium cell, Non-electrolyte, Osmotic pressure, Oxidation, Oxidation potential, Oxygen gas electrode, Primary cell, Redox electrode, Redox potential, Redox reaction, Reduction, Reduction potential, Reference electrode, Reversibility of cell, Salt bridge, Secondary cell, SHE, Single electrode, Solution pressure, Standard Hydrogen Electrode, Voltaic cell, watt, Working of the cell