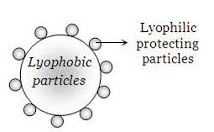
Science > Chemistry > Colloids > Associated Colloids (Micelles) In thisarticle, we shall study bout associated colloids :micelles). Multimolecular Colloids: Multimolecular colloids are those systems in which the dispersed phase particles are aggregates of many atoms or molecules. The particles in this colloidal solutions are held together by van der Wall’s forces. e.g. gold sol particles […]
Categories
Associated Colloids (Micelles)
- Post author By Hemant More
- Post date April 3, 2020
- 1 Comment on Associated Colloids (Micelles)
- Tags Alloys, Anionic surfuctants, Associated colloids, Cationic surfactants, Chemistry, Colloidal dispersions, Colloidal solution, Colloidal state, Colloids, Crystalloids, Dispersed phase, Dispersion medium, Emulsion, Foam, Gas in liquid solutions, Gas in solid solution, Gels, Liquid aerosols, Liquid in gas solutions, Liquid in liquid solutions, Liquid in solid solutions, Lyophilic sols, Lyophobic sols, Macromolecular colloids, MIcelles, Multimolecular colloids, Non-ionic surfactants, Solid aerosols, Solid foam, Solid in gas solutions, Solid in liquid solutions, Solid in solid solutions, Solid sols, Sols, Solution, Surfactants, Suspension, True solution