Science > Chemistry > Redox Reactions > Oxidation Number or Oxidation State Oxidation Number OR Oxidation State: The donation of electrons is called the oxidation and the gain of electrons is called the reduction. Oxidation and reduction can further be explained by a knowledge of “Oxidation number”. The oxidation state of an atom in its […]
Category: Chemistry
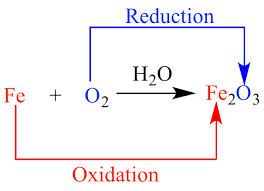
Science > Chemistry > Redox Reactions > Introduction to Redox Reactions In this article we shall study about redox reactions, in which both the oxidation and reduction reactions take place simultaneously. Oxidation: Old Concept: It is a process in which addition of oxygen takes place. 2Mg + O2 → 2MgO It is a […]
Applications of Colloids
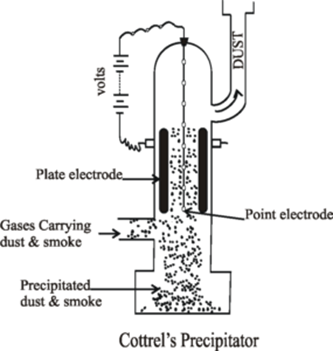
Science > Chemistry > Colloids > Applications of Colloids Natural Applications of Colloids: Blue Colour of Sky: When the light is incident on particles whose size is smaller than the wavelength of light, it is scattered. The blue colour of the sky is due to the scattering of light by small particles (dust particles along […]
Emulsions
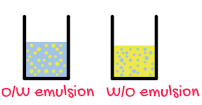
Science > Chemistry > Colloids > Emulsions A colloidal system in which both the dispersed phase as well as dispersion medium are immiscible or partially miscible liquids is called an emulsion. e.g. Milk, cod liver oil, oil paints, vanishing cream, cold creams, etc. are emulsions. Generally, one of the two liquids is water and the […]
Gels

Science > Chemistry > Colloids > Gels A gel is a colloidal system in which the dispersed phase is liquid and the dispersion medium is solid. e.g. when warm sol of gelatin is cooled, it sets to a semi-solid mass which is a gel. Jellies, jams, curd, butter, shoe polish, etc. are gels. The interior […]

Science > Chemistry > Colloids > Coagulation of Colloidal Solution The process of precipitation of colloidal particles due to aggregation of the particles is called coagulation or flocculation. Explanation: The presence of the same type of electric charge on colloidal particles causes repulsion and keep them in a suspended state. If by some means, the […]

Science > Chemistry > Colloids > Charge on Colloidal Particles The colloidal particles carry an electric charge. The most important property of colloidal solution is that all suspended particles possess either positive or a negative charge. i.e. they carry the same nature of the charge. The mutual forces of repulsion between similarly charged particles prevent […]
Properties of Colloids

Science > Chemistry > Colloids > Properties of Colloids In this article, we shall study the general, mechanical and optical properties of colloids. General Properties of Colloids: Heterogenous Character: The ultramicroscopic examination indicates that colloidal dispersion is a heterogeneous system consisting of a continuous dispersion medium and discontinuous disperse phase. Visibility: Colloidal particles cannot be seen […]

Science > Chemistry > Introduction to Chemistry > Naming of Chemical Compounds In this article, we shall study the method of naming of chemical compounds In a chemical reaction, the molecular composition changes and it is represented by a chemical equation. In a chemical equation, the various substances involved as reactants or products are written […]
There are many observable patterns in the physical and chemical properties of elements as we descend in a group or move across a period in the Periodic Table. The term periodicity is used to indicate that some characteristic properties occur in the periodic table after definite intervals, with a varying (gradual increase or decrease) magnitude. The periodic recurrence of elements having similar […]