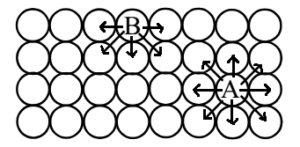
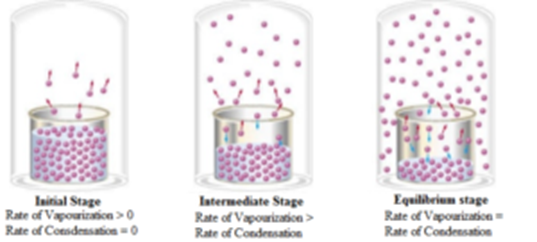
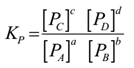Science > Chemistry > Surface Chemistry > Adsorption Adsorption is defined as the phenomenon in which there is the accumulation of one substance on the surface of the other substance. It can also be defined as the change in concentration at the interfacial layer between two phases of the system due to surface forces. OR It […]